Jianfan Hemoperfutor IFU: The Medical Device You Need to Know About! Ifu medical device: create ce-compliant instructions for use
If you are searching about EU MDR Medical Device Labeling Requirements-A Complete Guide you've visit to the right web. We have 25 Images about EU MDR Medical Device Labeling Requirements-A Complete Guide like IFU Otoscope | Download Free PDF | Medical Device | Disinfectant, ifu medical device Archives - Alysidia and also IFU Medical Device: Create CE-Compliant Instructions For Use. Here you go:
EU MDR Medical Device Labeling Requirements-A Complete Guide
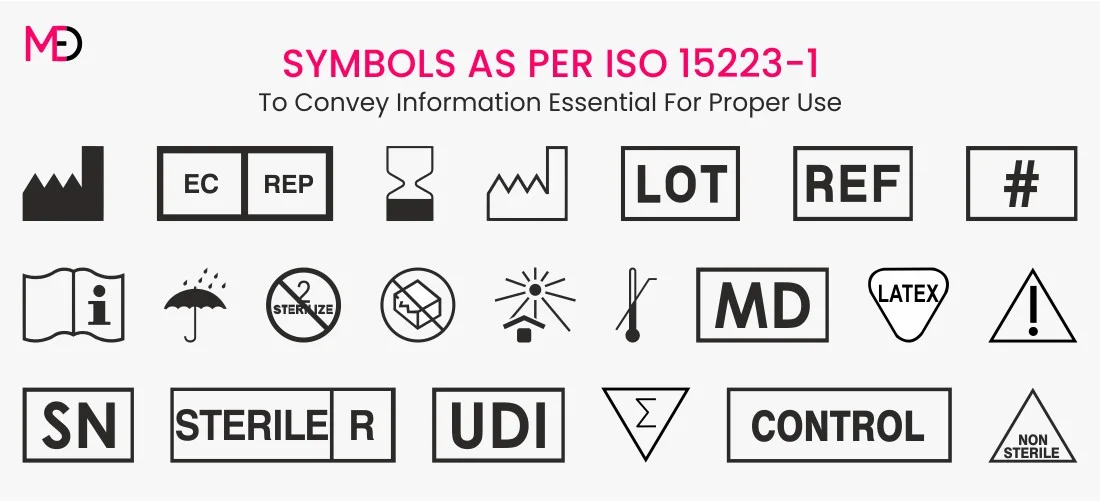 medicaldevicelicense.com
medicaldevicelicense.com
IFU Otoscope | Download Free PDF | Medical Device | Disinfectant
 www.scribd.com
www.scribd.com
Instruction For Use (IFU) – EU MDR 2017/745 – Easy Medical Device School
 school.easymedicaldevice.com
school.easymedicaldevice.com
Medical Devices - IFU Document (ENG-HUN)
 gtelocalize.com
gtelocalize.com
IFU Medical Device: Create CE-Compliant Instructions For Use
 instrktiv.com
instrktiv.com
Medical Device IFU & EIFU | Clin R
 clin-r.com
clin-r.com
IFU Medical Device: Create CE-Compliant Instructions For Use
 instrktiv.com
instrktiv.com
Essential IFU Medical Device Template For The U.S. Market
 instrktiv.com
instrktiv.com
IFU Checklist For Reusable Medical Devices | PDF
 www.slideshare.net
www.slideshare.net
IFU For Medical Devices, A Definitive Guide (EU & US)
 instrktiv.com
instrktiv.com
IFU For Medical Devices, A Definitive Guide (EU & US)
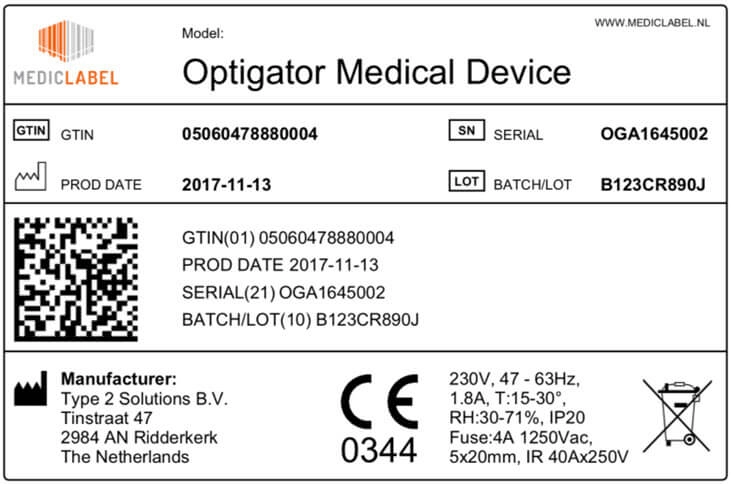 instrktiv.com
instrktiv.com
24cm Pressure Assisted Device | Merit Medical Safeguard 24cm
 www.manualsdir.com
www.manualsdir.com
ifu safeguard multilingual 24cm merit assisted
IFU ~ Medical Robotics Device On Behance
 www.behance.net
www.behance.net
Ifu Medical Device Archives - Alysidia
 alysidia.com
alysidia.com
ifu ifus
Instruction For Use (IFU) – EU MDR 2017/745 – Easy Medical Device School
 school.easymedicaldevice.com
school.easymedicaldevice.com
IFU Validation Is Not A Risk Reduction - Deviation 7
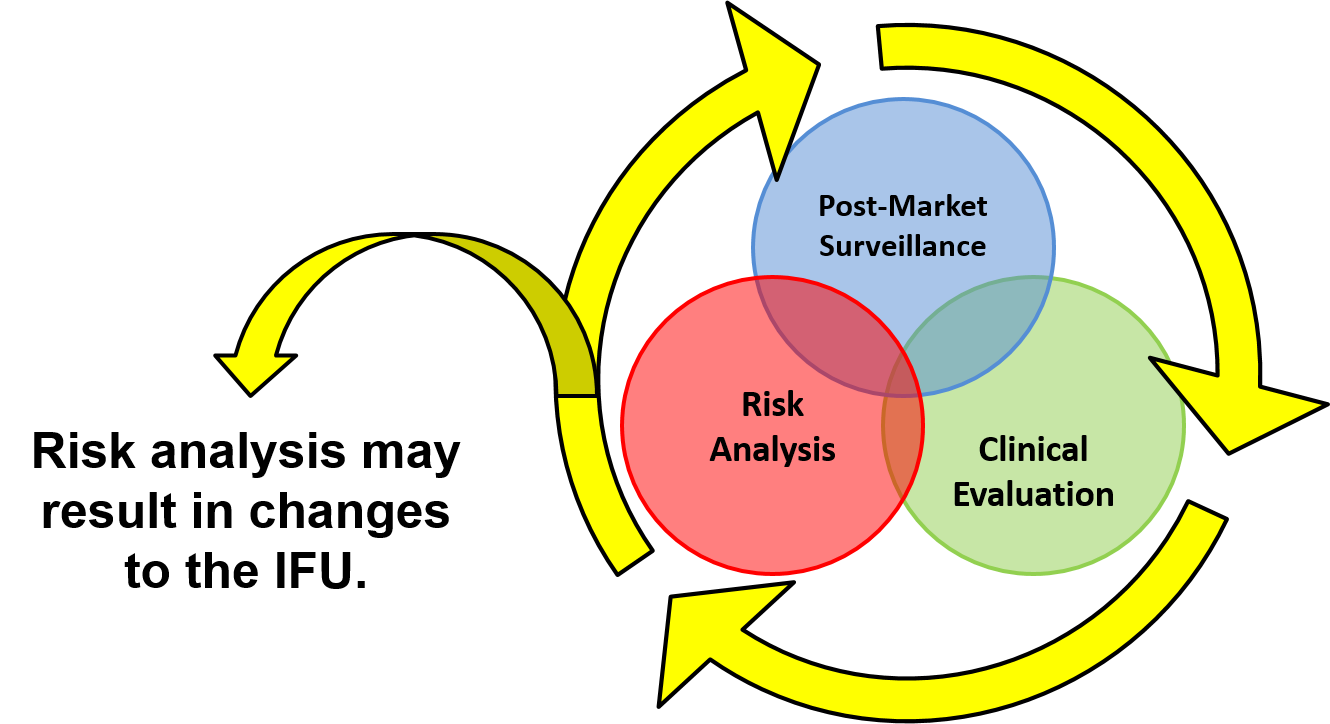 medicaldeviceacademy.com
medicaldeviceacademy.com
ifu post surveillance market validation risk medical pms clinical patient deviation reduction not device population devices approach based suitable expand
Instruction For Use (IFU) – EU MDR 2017/745 – Easy Medical Device School
 school.easymedicaldevice.com
school.easymedicaldevice.com
Medical Device Ifu Example At Nicole Ford Blog
 exyfxpdcz.blob.core.windows.net
exyfxpdcz.blob.core.windows.net
Medical Device IFU | Medical Device, Medical, Device Labels
 in.pinterest.com
in.pinterest.com
IFU For Medical Devices, A Definitive Guide (EU & US)
 instrktiv.com
instrktiv.com
Medical Device IFU & EIFU | Clin R
 clin-r.com
clin-r.com
E-IFU Solutions - Alysidia
 alysidia.com
alysidia.com
ifu
IFU Medical Device: Create CE-Compliant Instructions For Use
 instrktiv.com
instrktiv.com
Instruction For Use (IFU) – EU MDR 2017/745 – Easy Medical Device School
 school.easymedicaldevice.com
school.easymedicaldevice.com
IFU For Medical Devices, A Definitive Guide (EU & US)
 instrktiv.com
instrktiv.com
24cm pressure assisted device. Medical device ifu. Eu mdr medical device labeling requirements-a complete guide